Half 1. Clarify the features and roles of the next:
-
Notified Our bodies
-
Competent Authorities
-
HPRA
-
EMA
-
FDA
-
IMDRF
Notified our bodies
Notified our bodies are an organisation managing the evaluation and testing of merchandise, and are extra thought-about non-public our bodies reasonably than authorities managed (McDermott 2019). The European Fee produces a listing of organisational our bodies that serve to perform duties related to conformity evaluation procedures earlier than a product’s launch available on the market, particularly throughout third get together involvement. Producers can avail of the evaluation of compliance offered by the notified our bodies in relation to public curiosity. Notified our bodies should be knowledgeable by the related EU authorities of their vary of energy primarily based on rules declared in Decision 768/2008/EC. Notified our bodies can present their service for any operator and on any territory, no matter whether or not within the EU or not (Europa 2019).
Moreover, they’re required to behave in a clear, inclusive and non-biased method, and should rent workers with the perfect ability vary and expertise for his or her place in order that the conformity evaluation might be carried out with the related legal guidelines in thoughts. Confidentiality is of utmost significance for knowledge safety and as such the notified our bodies should make essential preparations to uphold that. Enough insurance coverage beforehand is critical in order that any losses throughout skilled exercise are lined or else should be assured by the related EU authorities as backed up. The notified our bodies at all times preserve the notifying authority and any market surveillance authorities knowledgeable of each exercise. As such, the notified physique should be monitored to make sure sure practices and laws are met. Accreditation organizations are accountable for checking their competence regularly; typically utilizing the EN ISO/IEC 17000 accreditation and requirements collection for serving to with the monitoring of conformity to related legislations (Europa 2019).
Competent Authorities
A Nationwide Competent Authority is one which has authorized capability to hold out sure pertained duties, mostly the monitoring of nationwide laws and statutes and making certain compliance happens (Europa 2019).
These policymaking companies are answerable for the introduction of nationwide laws. Not in contrast to departments in authorities, the companies even have appointed Ministers of Well being. A big number of advisory panels direct the authorities, and they’re of a extremely technical nature. As mirrored within the under Desk 1.1, roles and tasks consists of:
Desk 1.1 Roles and tasks of Nationwide Competent Authorities (McDermott 2019).
-
Analysis of purposes for drug advertising and marketing authorisations made both on to the nationwide authority or not directly by way of the centralised process to the EMEA;
|
-
Monitoring and approval of knowledge offered on leaflets, and promotional materials;
|
-
Nationwide licensing of medication/ Inspection and licensing of services;
|
-
Approval and licensing of medical trials/ Operation of market vigilance programs at nationwide stage.
|
The NCAs handle the authorization course of for medicines not adhering to the centralised process within the EU. The European Medicines Company intently cooperates with the nationwide competent authorities of the Member States of the European Union (EU) and the European Financial Space (EEA) answerable for human medicines (Europa 2019).
NCAs handle the laws for drugs within the EU, each human and veterinary. Their forces are mixed within the Head of Medicines Companies (HMA) discussion board. The European Fee and the EMA work along with the NCA leaders to warrant most collaboration and a extremely functioning regulatory community for European medicines. Quarterly conferences happen within the HMA in order to debate the perfect methods to uphold the community and give attention to areas akin to developments in IT and data alternate in order that the decentralised procedures and co-recognition are updated (Europa 2019).
HPRA
The Well being Merchandise Regulatory Authority (HPRA) is an Irish-based authority initially based because the Irish Medicines Board, which lined solely medicine, however now additionally contains medical units and merchandise (McDermott 2019).
Their function is to manage numerous well being merchandise, from medical units to medicine and even cosmetics, for the good thing about public security in relation to each human and veterinary practises. It’s a state company that assesses and controls well being associated produce created in Eire or exported abroad utilizing medical and scientific experience, with the intention of making certain well being merchandise work as envisioned. The improve in title was as a result of an growth of regulatory features that’s, extending to incorporate practices akin to medical trials, medical units, tissue and blood parts, beauty merchandise, animal welfare in relation to scientific trials, managed medicine and organ donations (HPRA 2019).
Furthermore, the HPRA produces firm licenses for creating and distributing medicines available on the market submit shut scrutiny of the standard and security, together with inspecting these organizations for compliance with nationwide laws and requirements. All health-related produce is continually monitored so any arising points are resolved instantly, and security and high quality data is at all times offered to make sure solely the perfect practices happen. The company is answerable for the regulation of any sort of organizations coping with drugs, together with numerous distribution and wholesale firms, regulating the manufacturing course of itself, different well being product facilities and any notified our bodies coping with medical units. The company additionally offers licenses for the utilization of animals in scientific or academic trials and inspects the method to warrant the appliance of the precept of 3R, that’s, discount, refinement and substitute (HPRA 2019).
EMA
The EMA is the European Medicines Company, which is a European regulatory physique for all pan-European firms. This company depends on a number of scientific committees which are of paramount affect in terms of the company’s well-being, as they’re the factor answerable for decision-making and issuing recommendation in relation to medicines. A large number of committees exist concentrating on various sectors akin to mirrored in under Determine 1.1:

Determine 1.1 EMA committees, adopted from (Europa 2019).
Any pharmacological firms that produce new high-end drugs for the EU should have their produce scientifically reviewed by the EMA. In 1995 the EMA was based to ensure supervision of medicines in Europe and essentially the most environment friendly allocation of scientific sources. Members of the EMA are typically consultants who function in numerous fields akin to scientific advisory teams, scientific committees, and dealing events are a part of nationwide evaluation groups who cope with drugs evaluation. Members are typically chosen primarily based on their experience within the subject, with many being put ahead by Nationwide Competent Authorities (NCAs) from states who’re members (Europa 2019).
There’s a rise of people who’re proficient in healthcare or are sufferers themselves which are turning into concerned on this course of too. Thus, the EMA makes use of these skilled opinions from its members to determine scientific tips, and as such are an correct illustration of present dialogue and views about biomedical science developments. Any drugs developer can then use these tips to ensure the very best high quality of medication and through the advertising and marketing authorization course of when proposing an utility. In addition to, the EMA offers recommendation associated to firms for the manufacturing of particular medicines. Thus, that is an especially helpful instrument for creating protected medicines of the very best high quality and making them obtainable to clients. The NCAs may also present such recommendation (Europa 2019).
FDA
The Meals and Drug Administration (FDA), which is the most important regulatory authority on a worldwide scale, is a US company who’s the primary supervisor for meals, units, cosmetics and medicines within the nation, in addition to being a department of the Division of Well being and Human Companies (HSS). It employs nearly 10,000 professionals who do a big portion of the safeguarding of the US residents well being. The President, together with the Senate’s consent, is who chooses a Commissioner for the duty of overseeing this company.
A few of the duties the FDA performs is creating regulatory insurance policies, establishing tips for the FDC act, inspecting merchandise and services for compliance with legislations, assessing and approving submissions of units and such, earlier than their market launch, safety towards false promoting in relation to well being merchandise, together with contributing to worldwide objectives involved with world harmonisation (McDermott 2019).
The FDA accommodates 9 Places of work and Facilities (FDA 2018).
The FDA acts on public well being and security and regulates fundamental produce like meals and cosmetics, but additionally tackles radiation emitting merchandise and every little thing associated to tobacco consumption. Along with that, the FDA is a serious element of the US’ anti-terrorism improvement FDA (2018).
The FDA has a really broad regulatory authority over the important thing areas already talked about, however it’s function might be similar to what another state our bodies are already answerable for. Therefore, it isn’t unusual for shoppers to be unsure about what regulatory company is related for his or her difficulty and whom to contact. Usually, the FDA is related to monitoring conventional product courses and their applicable subheadings (2018). Some main ones are mirrored in under Desk 1.2. This checklist isn’t exhaustive.
Desk 1.2. FDA product courses and subcategories (FDA 2018).
|
Product Class
|
Examples of Subheadings
|
|
Meals
|
Meals components, dietary dietary supplements
|
|
Medicine
|
Prescription and non-prescription medicine
|
|
Biologics
|
Vaccines, genetic produce
|
|
Medical units
|
Prosthetics, surgical implants
|
|
Radiation emitting merchandise
|
Mercury vapour lamps, x-ray merchandise, sunlamps
|
|
Cosmetics
|
Fragrance, pores and skin cleansers
|
|
Veterinary produce
|
Livestock and pet meals, veterinary medicine
|
|
Tobacco produce
|
Cigarettes, cigars
|
The FDA’s strategy to accepting market launch of merchandise varies relying on the product. It’s primarily based on relative threat to shopper well being and legal guidelines already in place by the FDA. Medical merchandise should have their advantages outweigh their dangers, as dangers will at all times be current in each regulated product, and thus it is crucial these judgements are taken critically. The one exception for a product to pose extra threat than profit is whether it is probably lifesaving.
Complicated merchandise like revolutionary medicine or prosthetics should be authorized as dependable and protected beforehand, whereas cosmetics or dietary dietary supplements might be marketed with out approval beforehand. The FDA does no testing or improvement itself. Common time for drug evaluation is round 6 months (FDA 2018).
The FDA produces data in relation to product remembers and updates the press and different companies constantly (FDA 2017).
IMDRF
The Worldwide Medical System Regulators Assignment help – Discussion board (IMDRF) was established within the October of 2011, however was envisioned earlier that 12 months as a discussion board for the dialogue of the synchronisation of medical gadget laws. It was established by a choice of medical gadget regulation representatives from everywhere in the globe, together with the World Well being Organisation, who volunteered to collect in Ottawa to debate the event of this discussion board. The purpose was to make use of the World Harmonization Job Power on Medical Gadgets (GHTF) as the idea was their concept and work from that to develop a extra synchronized system for worldwide medical gadget regulation (IMDRF 2019).
Regulatory officers are positioned on the IMDRF Administration Committee for the supervision of their insurance policies and techniques, together with managing membership and total Assignment help – Discussion board actions. Moreover, this Committee additionally manages the Working Teams that use the experience of a various vary of stakeholders from fields akin to academia, healthcare, sufferers/shoppers and business. A few of the Harmonization Initiatives already undertaken by IMDRF are in relation to working with affiliate organisations, that’s, APEC LSIF Regulatory Harmonization Steering Committee, the Pan American Well being Group (PAHO), and The Asian Harmonization Working Occasion (AHWP), all neglected by the Official Observers (IMDRF 2019).
A set variety of Official Observers from WHO or comparable regulatory authorities is delegated by the Administration Committee via a standard vote, primarily based on who would deem most respected to IMDRF on the time. Like Members, Official Observers are required to be extremely acquainted or knowledgeable about present IMDRF points however don’t partake within the technique of choice making (IMDRF 2019).
Affiliate Organisations are essential for IMDRF objectives. They’re regional or worldwide our bodies sharing the identical curiosity in upholding convergence of medical gadget regulatory practises, akin to by the tactical unfold of data and sources for the intention of creating dependable and protected medical on a worldwide scale, and as such IMDRF likes to determine actually good working relations with them (IMDRF 2019).
Half 2: In relation to Laws and Regulatory Technique, Clarify the objectives, rules and goals of regulation
Public well being safety is a prerogative of all laws. As such, the important thing factors to contemplate are the effectivity, goal, threat vs advantages, high quality and above all, security.
In relation to security, the product ought to ideally trigger no hurt, and the laws which are to be carried out ought to at all times take related measures to make sure product security (Tobin 2009).
In relation to medical units, the security issue is taken into account as essentially the most vital. A syringe aimed for blood assortment can function a very good instance. A blunt needle or malfunctioning affected person monitor can harm affected person well being and end in a vital state of affairs (WHO 2003).
Nevertheless, security can by no means be absolute as no gadget is ever risk-free, with some flaws not uncovered till a lot later. A main instance for this may be implants or prosthetics which spend appreciable period of time within the human physique, solely to have a critical unpredicted defect years later. Gadgets can fail primarily based on components as a result of distinctive circumstances in a affected person, or in an unpredictable method not associated to something. Threat evaluation is a course of the place one identifies the possibilities of a tool failing with hazardous unwanted effects (WHO 2003).
A clinically efficient gadget is one which produces the specified impact (WHO 2003). Thus, one should additionally set up the supposed utilization for the product (Tobin 2009). If the product is meant for ache reduction, it’s essential for the developer to have medical outcomes backing it up together with inflicting precise ache reduction. Effectiveness is identical as efficacy for present medical ranges of a managed atmosphere.
When speaking about efficacy, medical units should not compromise on the well-being of sufferers or another individuals concerned. Thus, it’s of utmost significance the danger vs advantages are at all times calculated, with the priorities at all times positioned on the profit facet. That is known as threat administration. There exists a protocol by the Worldwide Group for Standardization (ISO) for help with such. It offers a framework for producers for the analysis and administration of threat in relation to the medical gadget business (WHO 2003).
Strong proof akin to enter from sufferers through the threat/profit evaluation should be offered beforehand so that every one choices might be made in essentially the most rational and knowledgeable method (FDA 2016).
High quality is one other facet to contemplate. Security and health for goal are the 2 main traits that one would take into account for a product to be seen as of top quality. Nevertheless, there are different main parameters of a high quality product and providers akin to reliability and consistency. High quality programs serve the aim to realize applicable stage of the described parameters by many firms. Companies in different industries can use high quality programs on voluntary foundation. This isn’t the case for producers of potential high-risk medical units and medicines. Such companies are legally obliged to have applicable high quality programs in place. Furthermore, there are laws that outline specifics for the standard programs to be utilized inside such companies (Tobin 2009).
In its personal flip, implementation of high quality administration requires sources, procedures, organizational construction, tasks and processes which are outlined by high quality system. Necessities of high quality programs can affect all phases of medical units life span and high quality programs laws could embrace completely different elements of strategies and controls in such areas as packaging, storage, labelling, manufacturing, servicing, and so on. The Worldwide Group defines the requirements of worldwide high quality system for medical units for Standardization (ISO). Furthermore, the strict high quality requirements in manufacturing end in Good Manufacturing Apply. It is a manufacturing system with excessive stage of requirements and controls. GMP helps to cut back non-conformity, ensures higher stage of merchandise efficiency and security in addition to helps to keep up consistency of high quality. The key advantage of high quality programs is their proactive strategy versus the reactive nature of “inspection-rejection” programs in manufacturing course of (WHO 2003).
Different two vital parts of any high quality system is documentation and other people. For instance, applicable and well timed documentation is a key factor for any audit or inspection. And, after all, any system is barely as efficient because the professionals that use it. Therefore, you will need to be sure that each related worker is conscious and perceive his or her enter and affect to high quality.
When speaking about fundamental regulatory technique, under Determine 2.1 displays its main focus parts: product improvement and manufacture in addition to market vigilance.
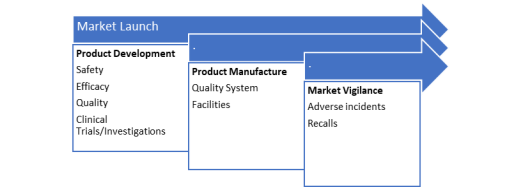
Determine 2.1. Parts of the Regulatory Technique, tailored from (McDermott 2019).
At a product improvement stage, product traits akin to security and effectivity must be thought-about. The builders should generate applicable knowledge as a way to current it for authorities evaluation. Regulatory authorities grant authorisation for advertising and marketing and gross sales if the info evaluation is passable. They’re additionally concerned in approval of medical trials and investigations which are wanted for security and effectiveness assessments. Furthermore, supplies, high quality programs and requirements might be additionally assessed the place applicable (Tobin 2009).
Product manufacture is one other focus space of regulatory technique. The manufacturing websites should be registered with authorities and might be inspected regularly by related authorities. Such audits and inspections intention to confirm that hygienic requirements are of a sure stage whereas environment friendly high quality programs are in place (Tobin 2009).
The third space of focus in regulatory technique is post-market surveillance/vigilance. It is just when the security and efficiency of medical units are constantly assessed that one can make certain that they’re of the required requirements. Therefore, submit market surveillance helps to observe and evaluation medical units in use. Beneath Determine 2.2 exhibits two main actions of surveillance course of:
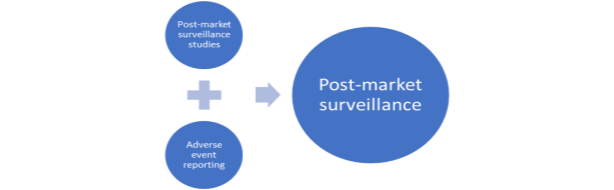
Determine 2.2 Publish Market Surveillance, tailored from (WHO 2003).
Publish-market surveillance research are characterised by knowledge assortment of particular and applicable data as situation of product approval or re-confirmation its requirements when required. Opposed occasion reporting is characterised by registration and investigation of opposed occasions of medical gadget use that may result in its modification/recall by authorities (WHO 2003).
References
-
Europa (2019) Nationwide competent authorities, obtainable: https://www.ema.europa.eu/en/partners-networks/eu-partners/eu-member-states/national-competent-authorities-human [accessed 15 Jan 2019].
-
Europa( 2019) Notified our bodies, obtainable: https://ec.europa.eu/growth/single-market/goods/building-blocks/notified-bodies_en [accessed 15 Jan 2019].
-
Europa (2019) The European regulatory system for medicines, obtainable: https://www.ema.europa.eu/documents/leaflet/european-regulatory-system-medicines-european-medicines-agency-consistent-approach-medicines_en.pdf [accessed 15 Jan 2019].
-
FDA (2018) Concerning the FDA Group Charts, obtainable: https://www.fda.gov/AboutFDA/CentersOffices/OrganizationCharts/default.htm [accessed 14 Jan 2019].
-
FDA (2018) About FDA Steering Paperwork, obtainable: https://www.fda.gov/RegulatoryInformation/Guidances/default.htm [accessed 13 Jan 2019].
-
FDA (2017) About FDA Product Approval, obtainable: https://www.fda.gov/NewsEvents/ProductsApprovals/ucm106288.htm [accessed 14 Jan 2019].
-
FDA (2018) What does FDA regulate? https://www.fda.gov/AboutFDA/Transparency/Basics/ucm194879.htm [accessed 14 Jan 2019].
-
FDA (2018) What We Do, obtainable: https://www.fda.gov/AboutFDA/WhatWeDo/default.htm [accessed 14 Jan 2019].
-
FDA (2016) Components to Contemplate Relating to Profit-Threat in Medical System Product Availability, Compliance, and Enforcement Choices, obtainable: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm506679.pdf [accessed 14 Jan 2019].
-
FDA (2018) Remembers, Market Withdrawals, & Security Alerts, obtainable: https://www.fda.gov/Safety/Recalls/default.htm [accessed 13 Jan 2019].
-
HPRA (2019) About Us, obtainable: https://www.hpra.ie/homepage/about-us [accessed 15 Jan 2019].
-
IMDRF (2019) About IMDRF, obtainable: http://www.imdrf.org/about/about.asp [accessed 1 Jan 2019].
-
IMDRF (2019) Worldwide Medical System Regulators Assignment help – Discussion board, obtainable: http://www.imdrf.org/index.asp [accessed 15 Jan 2019].
-
McDermott, O. (2019) ‘Regulatory Affairs –Introduction’, BST109: B.Sc in Science & Know-how, 15 Jan, AUA, unpublished.
-
Tobin J.J (2009) ‘Regulatory Compliance’, BST109, Nationwide College of Eire, Galway, unpublished.
-
WHO (2003) Medical gadget Laws, World overview and guiding rules, obtainable: https://www.who.int/medical_devices/publications/en/MD_Regulations.pdf [accessed 13 Jan 2019].



